Ozempic Alternatives Facing FDA Scrutiny: Impact On Access And Availability
Table of Contents
FDA Scrutiny of Ozempic and Similar Medications: What's Happening?
The FDA's heightened scrutiny of Ozempic and its alternatives stems from several key concerns. Safety is paramount; potential side effects, including pancreatitis and gallbladder problems, require careful monitoring. Furthermore, the widespread off-label use of these medications for weight loss, initially approved for type 2 diabetes management, raises concerns about both efficacy and safety in broader patient populations. The FDA's regulatory process involves a thorough review of existing data, potentially leading to warnings, restrictions on prescribing practices, or even market withdrawals. This uncertainty creates ripples throughout the healthcare system.
Specific Examples of FDA Actions:
- Semaglutide (Wegovy): The FDA has issued warnings regarding potential side effects and is closely monitoring post-market surveillance data. [Link to relevant FDA announcement]
- Liraglutide (Saxenda): The agency has conducted reviews regarding its use in specific patient populations, resulting in updated prescribing information. [Link to relevant FDA announcement]
- Other GLP-1 Receptor Agonists: The FDA is actively reviewing data on other similar medications to assess their long-term safety and efficacy profiles. [Link to relevant FDA announcement, if available]
Impact on Access and Availability of Ozempic Alternatives
The increased regulatory scrutiny is already impacting the accessibility of these medications. Several potential consequences are emerging:
- Shortages: Increased demand coupled with potential production restrictions or limitations due to FDA actions could lead to drug shortages.
- Increased Cost: Restrictions or changes in manufacturing could drive up the cost of these medications, making them less affordable for many patients.
- Changes to Prescribing Practices: Healthcare providers may face stricter guidelines for prescribing these medications, potentially limiting access for some patients.
- Restrictions on Patient Eligibility: The FDA may impose stricter eligibility criteria, further limiting who can receive these treatments.
Challenges for Patients:
Patients relying on these medications for weight management face significant challenges. Those struggling with obesity or related conditions may experience difficulties accessing treatment, leading to potential health setbacks. The uncertainty surrounding availability can lead to anxiety and disruption in their treatment plans.
Impact on Healthcare Providers:
Healthcare providers are navigating a rapidly changing landscape. They face the challenge of adapting to new guidelines, managing patient expectations, and ensuring optimal care in the face of potential medication limitations. Concerns about patient access and the availability of effective alternative treatments are paramount.
Exploring Alternative Weight Management Strategies
While Ozempic and similar medications have proven effective for some, a holistic approach to weight management is crucial. Lifestyle modifications, including dietary changes and regular exercise, remain cornerstone strategies. A balanced diet, focusing on whole foods and portion control, coupled with a tailored exercise regimen, are critical for long-term success.
Non-Pharmaceutical Approaches:
- Dietary Changes: Consulting a registered dietitian to develop a personalized nutrition plan.
- Exercise Programs: Engaging in regular physical activity, including aerobic exercises and strength training.
- Support Groups: Joining weight loss support groups for motivation and peer support.
- Cognitive Behavioral Therapy (CBT): Addressing underlying psychological factors contributing to weight management challenges.
The Future of Ozempic and Similar Medications: Looking Ahead
The long-term implications of the FDA scrutiny are still unfolding. The future of this class of medications remains uncertain, although it’s likely that stricter regulations and more stringent safety monitoring will be implemented. This could lead to the development of new, safer medications or alternative treatment approaches in the weight management field. The focus is shifting towards personalized medicine, addressing the unique needs of individual patients.
Conclusion: Understanding the FDA's Impact on Ozempic Alternatives – A Call to Action
The FDA's increased scrutiny of Ozempic and its alternatives is significantly impacting access and availability. Patients and healthcare providers must stay informed about the evolving regulatory landscape. It's vital to understand the potential benefits and risks associated with these medications and to explore alternative weight management strategies. Finding suitable Ozempic alternatives, or indeed, navigating the Ozempic alternative landscape effectively, requires open communication with healthcare professionals. Remember to discuss your weight management goals and explore all available options with your doctor to develop a personalized and safe plan for your individual needs.

Featured Posts
-
 Wyoming Reports Death Of Second Colorado Gray Wolf
May 22, 2025
Wyoming Reports Death Of Second Colorado Gray Wolf
May 22, 2025 -
 Abn Amro Ziet Occasionverkoop Flink Toenemen Analyse Van De Markt
May 22, 2025
Abn Amro Ziet Occasionverkoop Flink Toenemen Analyse Van De Markt
May 22, 2025 -
 Sharath Kamal Bids Adieu Wtt Star Contender Chennai 2025
May 22, 2025
Sharath Kamal Bids Adieu Wtt Star Contender Chennai 2025
May 22, 2025 -
 Dutch Central Bank Probes Abn Amros Bonus System
May 22, 2025
Dutch Central Bank Probes Abn Amros Bonus System
May 22, 2025 -
 Raw Video Pub Landladys Uncensored Reaction To Employees Notice
May 22, 2025
Raw Video Pub Landladys Uncensored Reaction To Employees Notice
May 22, 2025
Latest Posts
-
 Core Weave Stock Crwv Reacts To Nvidias Strategic Investment
May 22, 2025
Core Weave Stock Crwv Reacts To Nvidias Strategic Investment
May 22, 2025 -
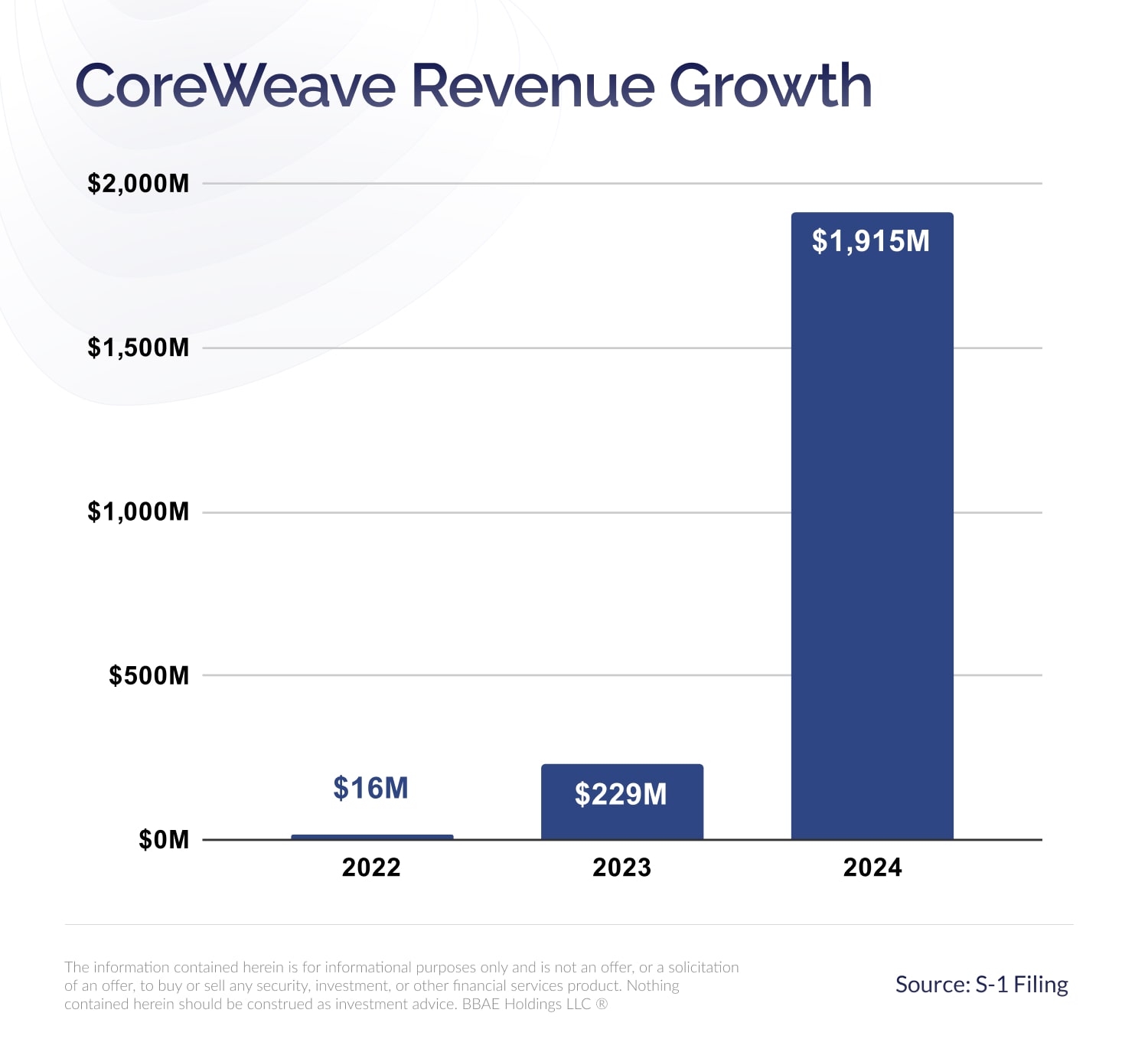 Core Weave Crwv Stock Market Performance Explaining Todays Gains
May 22, 2025
Core Weave Crwv Stock Market Performance Explaining Todays Gains
May 22, 2025 -
 Why Did Core Weave Crwv Stock Price Rise Today An Analysis
May 22, 2025
Why Did Core Weave Crwv Stock Price Rise Today An Analysis
May 22, 2025 -
 Core Weave Inc Crwv Stock Soars Reasons Behind The Increase
May 22, 2025
Core Weave Inc Crwv Stock Soars Reasons Behind The Increase
May 22, 2025 -
 Jim Cramers Investment Thesis Core Weave Crwv And The Ai Revolution
May 22, 2025
Jim Cramers Investment Thesis Core Weave Crwv And The Ai Revolution
May 22, 2025
